
The results were recorded after all of the solutions were mixed together. There were many areas where an error could have occurred in the lab. Regardless of what these ions are paired with, they will always be aqueous. For example, nitrates, acetates, ammonium and group 1 salts.
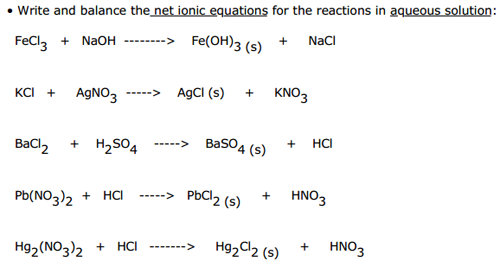
The exceptions are based on the ions that they are paired with. This is because most solubility rules have exceptions. The first ion in the compound determines whether a substance is insoluble or soluble. When the double replacement reaction occurs the "partners" switch. The patterns that each cation or anion had contributed to the statement. General statements about specific cations and anions were concluded because of the data from the experiment.

Carbon dioxide gas was produced and bubbles were observed during the lab. Lastly, the solutions that gave off gas (bubbles) are referred to as gas formation reactions. By getting rid of the H+ and OH-, it neutralizes the reaction. Neutralization reactions eliminate the acid (H+) and base (OH-) to form water as a product. Neutralization reactions are the reactions that produces water and a salt. Those reactions are referred to as precipitation reactions. Most of the reactions in the experiment formed a precipitate.

However, when two aqueous solutions are mixed the anions and cations come together and form a precipitate, or gas. There are 3 types of metathesis reactions: precipitation reactions, neutralization reactions, and gas formation reactions. The hydration of water, due to water polarity, helps keep the ions separated in the solution. The cations (positive) and anions (negative) are attracted to each other. The ions switch partners because opposites attract. It allows us to see what caused the solids, liquids, or gases to form.Ī metathesis reaction is when cations and anions switch partners (double replacement reaction). A net ionic equation shows exactly what reactants are involved in the reaction. Strong acids and bases alos dissociate and are written in ions. The complete ionic equation shows all of the ions that dissociate and the compounds that stay together (solids, liquids, & gases). Spectator ions are the ions that do not change at the end of the reaction. Therefore, more ions are available to move around in the solution.Ī net ionic equation leaves the spectator ions out. Weak electrolytes cannot conduct as much as strong electrolytes because the concentration of ions are higher in strong electrolytes. An electrolyte is a substance that can conduct electric currents, the flow of electrons. When a substance is soluble it is a strong electrolyte because the concentration of ion in the solution is high because they were spilt apart by water. Weak electrolytes are slightly ionized because the attraction to the water's partial charges are not as strong in weak electrolytes. Depending on the strength of the electrolytes, some completely ionized when dissolved in water and others slightly ionize. Therefore, two aqueous products results in a no reaction. The reactants remain the same and nothing new formed. This is because the aqueous solutions that it began had the ions spilt up and at the end of the reaction the ions are still spilt up and floating around. In the hydration of polar solute molecules the negative ends of the polar solute molecules are attracted to the hydrogens (partially positive) and the positive ends of the polar solute molecule are attracted to the oxygen (partially negative).Ī reaction does not react if the end products are aqueous.
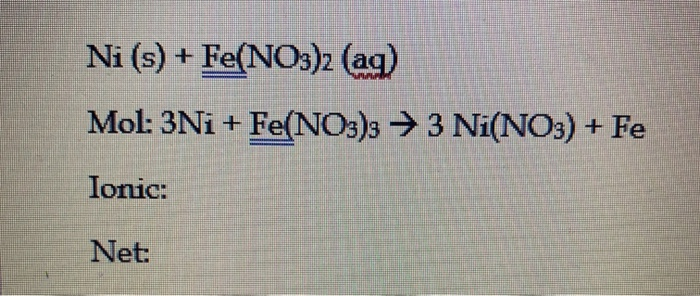
In the hydration of ionic solute molecules positive ions are attracted to the oxygen end of the water and negative ions are attracted to the hydrogen end of the water. It leave oxygen with a partially negative charge and hydrogen with a partially positive side. Oxygen is more electronegative than hydrogen and it results in an unequally sharing of the electrons. Water is known as the universal solvent because of its polarity. Polarity determines whether the solute will dissolve into the solvent. This is due to the idea that "like dissolves like." When a nonpolar compound is mixed in a polar solution the mixture will form a precipitate because the polar solution cannot dissolve nonpolar compounds and vice versa. Some of the reactant that were mixed together in the lab did not result in a precipitate, color change, or generation of a gas.


 0 kommentar(er)
0 kommentar(er)
